By Study Rhino – Learning Made Easy!
The Periodic Table of Elements is one of the most important tools in the world of science. It’s a chart that organizes all known chemical elements based on their atomic number, electron configurations, and recurring chemical properties. Whether you’re a student stepping into chemistry for the first time or a curious learner brushing up on your science skills, understanding the periodic table is a must. In this guide, we’ll break down everything you need to know—clearly and simply.
- What Is the Periodic Table?
At its core, the periodic table is a chart that arranges elements in a systematic way. Each element is represented by a box containing its chemical symbol (like H for hydrogen or O for oxygen), its atomic number (number of protons), and its atomic mass.
But it’s more than just a list. The periodic table reveals the patterns in element behavior and helps chemists predict how elements will react in different situations.
- The History Behind It
The periodic table wasn’t built in a day. In fact, several scientists contributed to its development. The most famous name associated with it is Dmitri Mendeleev, a Russian chemist who published the first widely recognized version in 1869.
Mendeleev arranged elements by increasing atomic mass and noticed that certain types of elements appeared at regular intervals. This “periodicity” led him to organize elements into groups with similar properties. Amazingly, he even left gaps in his table for elements that hadn’t yet been discovered—and many of those predictions turned out to be correct!
Later, the table was refined to arrange elements by atomic number (number of protons), thanks to the work of Henry Moseley, which is how the modern periodic table is arranged today.
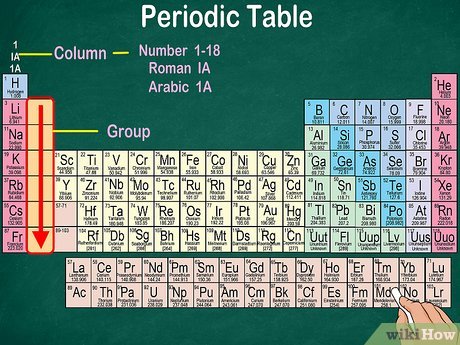
- Structure of the Periodic Table
The table has rows and columns, known as periods and groups, respectively.
- Periods (Horizontal Rows)
There are 7 periods in the table. Each period represents a new electron shell being filled. As you move left to right across a period, elements become less metallic and more non-metallic in character.
- Groups (Vertical Columns)
There are 18 groups (numbered 1 to 18). Elements in the same group share similar properties because they have the same number of electrons in their outer shell (called valence electrons). For example, Group 1 elements (alkali metals) are all very reactive with water.
- Categories of Elements
The periodic table can be divided into several types of elements:
- Metals
- Found on the left and center.
- Good conductors of heat and electricity.
- Malleable and ductile.
- Tend to lose electrons in chemical reactions.
- Example: Iron (Fe), Copper (Cu), Gold (Au)
- Non-metals
- Found on the right side.
- Poor conductors.
- Brittle when solid.
- Tend to gain or share electrons.
- Example: Oxygen (O), Carbon (C), Nitrogen (N)
- Metalloids
- Found along the zigzag line (stair-step line).
- Have properties of both metals and non-metals.
- Example: Silicon (Si), Arsenic (As)
- Special Groups on the Periodic Table
Some groups have unique names because of their similar characteristics:
- Group 1: Alkali Metals
- Extremely reactive, especially with water.
- Soft metals.
- One valence electron.
- Example: Sodium (Na), Potassium (K)
- Group 2: Alkaline Earth Metals
- Less reactive than alkali metals but still reactive.
- Two valence electrons.
- Example: Magnesium (Mg), Calcium (Ca)
- Groups 3–12: Transition Metals
- Strong, shiny, and good conductors.
- Often used in building materials and electronics.
- Example: Iron (Fe), Nickel (Ni), Zinc (Zn)
- Group 17: Halogens
- Very reactive non-metals.
- Seven valence electrons.
- Often form salts with metals.
- Example: Chlorine (Cl), Fluorine (F)
- Group 18: Noble Gases
- Very stable and unreactive.
- Complete outer electron shells.
- Example: Helium (He), Neon (Ne), Argon (Ar)
- The Modern Layout: Blocks of the Table
The periodic table is also divided into blocks based on electron configuration:
- s-block: Groups 1 and 2 + Helium
- p-block: Groups 13 to 18
- d-block: Transition metals
- f-block: Lanthanides and Actinides (placed below the main table)
This organization helps chemists understand how electrons are arranged in atoms and predict chemical behaviors.
- Trends in the Periodic Table
Understanding the trends or “periodicity” across the table makes it easier to predict an element’s behavior.
- Atomic Radius
- Size of an atom.
- Decreases across a period (left to right).
- Increases down a group.
- Ionization Energy
- Energy needed to remove an electron.
- Increases across a period.
- Decreases down a group.
- Electronegativity
- Tendency to attract electrons.
- Increases across a period.
- Decreases down a group.
These patterns are key for predicting bonding and reactions.
- The Importance of the Periodic Table
Why should students care about the periodic table? Here’s why it matters:
- It’s a roadmap to all known substances.
- Predictive power: You can guess how an unknown element might react.
- Foundation for chemistry: Bonding, reactions, equations—all start here.
- Real-world relevance: Elements are used in medicine, technology, agriculture, and more.
Whether you’re studying acids and bases, oxidation reactions, or electricity, the periodic table is always your companion.
- Fun Facts About the Periodic Table
- Element 118 (Oganesson) is named after Russian physicist Yuri Oganessian.
- Gold (Au) and Silver (Ag) come from Latin names: Aurum and Argentum.
- The periodic table has room to grow as scientists continue to discover new synthetic elements in labs.
- The rarest naturally occurring element on Earth is Astatine.
- Tips to Learn the Periodic Table
Here are some effective ways to memorize or understand it better:
- Use Mnemonics
For Group 1: “Hi He Likes Beer But Could Not Offer Full Nine Snacks”
(H for Hydrogen, He for Helium, etc.)
- Color Code It
Use colors to highlight metals, non-metals, gases, and specific groups.
- Interactive Apps
Try apps like “Ptable” or “The Elements” for an engaging learning experience.
- Flashcards
Old-school, but effective—quiz yourself on atomic numbers, symbols, and properties.
- Conclusion
The periodic table is more than a classroom chart—it’s a universal tool that helps scientists unlock the secrets of matter. Understanding how it’s organized, what each element represents, and how trends work can help you master chemistry with confidence.
At Study Rhino, we believe that complex science can be made simple—and even fun! So whether you’re studying for an exam or just exploring your curiosity, keep the periodic table close—it’s your key to the building blocks of the universe.